Data Extractor
Data Extractor is a powerful optional module for viewing, searching and filtering comet data that has been scored with Comet Assay IV and stored in an Oracle database. This system is commonly utilized in GLP-regulated environments to ensure study data security and integrity.
Data Extractor also gives authorized users the power to approve and reject data, archive studies and export result and audit data. For full genetox study management, including experimental set-up and automatic reporting, please visit our Cyto Study Manager solution.
Features of Data Extractor:
Control your data - Within Data Extractor you can browse, sort and filter your result and audit data within the Oracle database. What’s more, you can also review and approve or reject your data.
Flexible and efficient data transfer and processing - Users can export study data to Excel, then analyze it with the spreadsheet generator or any other data processing/reporting tool. Result files and images can be exported in batches. This means you can export all the results for a study in one group! These files can be named automatically for easy identification.
Increase your data security - Our solution allows for ultimate portability, integration into existing data management systems and security of data.
Verify your scoring process - Whether you want to “spot check” or “verify” a process, you can access images of scored cells via Data Extractor. Each cell image is stored with result data and information on exactly how the user scored the cell. These images can be opened in Comet Assay IV and re-scored to verify the data.
Easily find specific study data - Data Extractor makes it easy to locate any past data for review or statistical analysis. Data can be sorted and grouped quickly by clicking on column headings, and the “filter builder” offers powerful data filtering capabilities allowing the creation of complex search criteria for selecting data in any conceivable way. Filters can also be saved for future use.
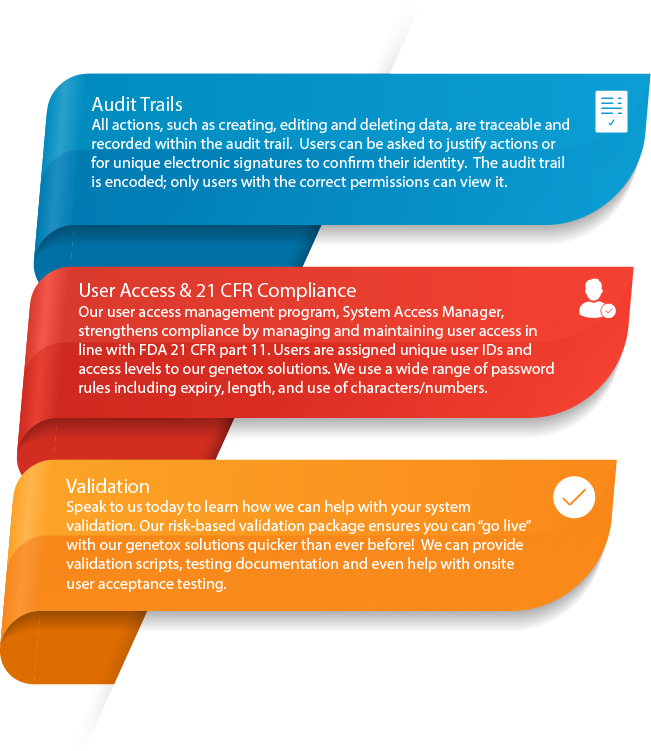
Archive your data in accordance with OECD guidelines - Sophisticated electronic signatures and archiving tools allow you to annotate data as “archived” within the database. This not only helps ensure the integrity of your data by avoiding any potentially dangerous export and purge operations, but also means you can still view the study and reconstruct reports for auditing and verification.
Ensure regulatory compliance - System Access Manager allows complete 21 CFR Part 11 compliance. All audit data collected during the use of Comet Assay IV can be viewed by authorised users within the Data Extractor and exported, if desired.
You work to GLP and so do our genetox solutions…
All of Instem’s genetox solutions are designed with reference to the OECD and S2(R1) guidelines. We ensure our solutions are fully compliant with the principles of Good Laboratory Practice (GLP) and the FDA 21 CFR part 11 rule on electronic signatures.